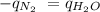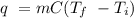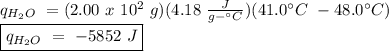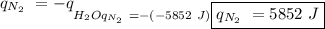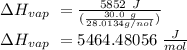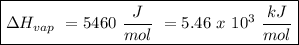Chemistry, 23.07.2019 02:20 bryanmcmillianjr
The heat of vaporization of a liquid (δhvap) is the energy required to vaporize 1.00 g of the liquid at its boiling point. in one experiment, 30.0 g of liquid nitrogen (boiling point = −196°c) is poured into a styrofoam cup containing 2.00 × 102 g of water at 48.1°c. calculate the molar heat of vaporization of liquid nitrogen if the final temperature of the water is 41.0°c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1
You know the right answer?
The heat of vaporization of a liquid (δhvap) is the energy required to vaporize 1.00 g of the liquid...
Questions

Social Studies, 24.02.2022 05:30



Mathematics, 24.02.2022 05:30

Mathematics, 24.02.2022 05:30



Biology, 24.02.2022 05:30




Mathematics, 24.02.2022 05:30


Mathematics, 24.02.2022 05:30

History, 24.02.2022 05:30

Health, 24.02.2022 05:30

Computers and Technology, 24.02.2022 05:30


History, 24.02.2022 05:40