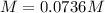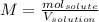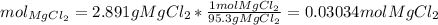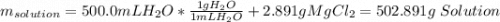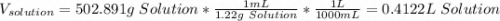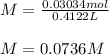Chemistry, 22.07.2019 22:20 GEEKLIFE6598
Question5 of 20if 2.891 g mgcl2 is dissolved in enough water to make 500.0 ml of solution, what is the molarity of the magnesium chloride solution? the molecular mass of magnesium chloride is 95.3 g/mol and the density of the solution is 1.22 g/ml.1.518 x 10-2 m5.782 x 10-3 m6.073 x 10-2 m0.5505 m5.782 m

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
Question5 of 20if 2.891 g mgcl2 is dissolved in enough water to make 500.0 ml of solution, what is t...
Questions

Social Studies, 20.05.2021 22:40

English, 20.05.2021 22:40



Mathematics, 20.05.2021 22:40


Mathematics, 20.05.2021 22:40

Mathematics, 20.05.2021 22:40


Physics, 20.05.2021 22:40

English, 20.05.2021 22:40

Mathematics, 20.05.2021 22:40



Mathematics, 20.05.2021 22:40


Chemistry, 20.05.2021 22:40

Physics, 20.05.2021 22:40

Mathematics, 20.05.2021 22:40