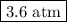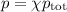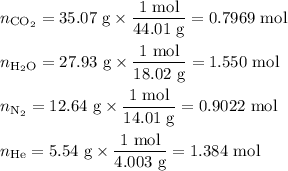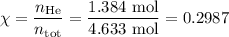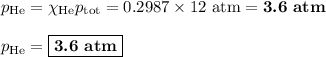Chemistry, 22.07.2019 05:10 tiwaribianca475
Amixture contains 35.07 grams of carbon dioxide, 27.93 grams of water vapor, 12.64 grams of nitrogen, and 5.54 grams of helium. the total pressure of the system is 12 atm, what is the partial pressure of the helium?
a. 0.88 atm
b. 0.82 atm
c. 0.073 atm
d. 0.068 atm

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Asolution at 25 degrees celsius is 1.0 × 10–5 m h3o+. what is the concentration of oh– in this solution?
Answers: 1

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

You know the right answer?
Amixture contains 35.07 grams of carbon dioxide, 27.93 grams of water vapor, 12.64 grams of nitrogen...
Questions

History, 23.09.2021 19:40


Mathematics, 23.09.2021 19:50

English, 23.09.2021 19:50



Mathematics, 23.09.2021 19:50

Advanced Placement (AP), 23.09.2021 19:50



English, 23.09.2021 19:50


Mathematics, 23.09.2021 19:50

English, 23.09.2021 19:50



History, 23.09.2021 19:50