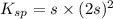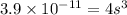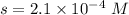What is the molar solubility of calcium fluoride ( caf2) in water? the solubility-product constant for caf2 is 3.9 ⋅ 10-11 at 25 °c. what is the molar solubility of calcium fluoride ( caf2) in water? the solubility-product constant for caf2 is 3.9 10-11 at 25 °c. 3.4 ⋅ 10-4 8.8 ⋅ 10-6 3.1 ⋅ 10-6 1.3 ⋅ 10-11 2.1 ⋅ 10-4

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
What is the molar solubility of calcium fluoride ( caf2) in water? the solubility-product constant...
Questions







Spanish, 27.05.2020 22:06

Mathematics, 27.05.2020 22:06

Social Studies, 27.05.2020 22:06

Mathematics, 27.05.2020 22:06

History, 27.05.2020 22:06


Physics, 27.05.2020 22:06

Mathematics, 27.05.2020 22:06


Biology, 27.05.2020 22:06

Mathematics, 27.05.2020 22:06

Mathematics, 27.05.2020 22:06

Mathematics, 27.05.2020 22:06

![K_{sp}=\left[Ca^{2+} \right]\left[F^{-} \right]^2](/tpl/images/0110/5296/1ebe5.png)