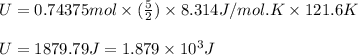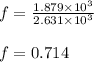Chemistry, 20.07.2019 02:30 aubriebv2020
Suppose 23.8 g of oxygen (o2) is heated at constant atmospheric pressure from 27.4°c to 149°c. (a) how many moles of oxygen are present? (take the molar mass of oxygen to be 32.0 g/mol) (b) how much energy is transferred to the oxygen as heat? (the molecules rotate but do not oscillate.) (c) what fraction of the heat is used to raise the internal energy of the oxygen?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 20:40
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
Suppose 23.8 g of oxygen (o2) is heated at constant atmospheric pressure from 27.4°c to 149°c. (a) h...
Questions




Mathematics, 01.06.2021 04:20


English, 01.06.2021 04:20





Physics, 01.06.2021 04:20


Physics, 01.06.2021 04:20


Mathematics, 01.06.2021 04:20




Mathematics, 01.06.2021 04:20

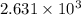
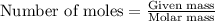
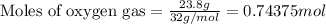
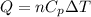
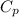 = specific heat capacity at constant pressure =
= specific heat capacity at constant pressure = 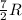 (For diatomic gas)
(For diatomic gas) = change in temperature =
= change in temperature = 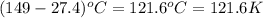
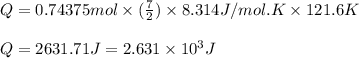
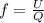
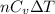
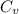 = specific heat capacity at constant pressure =
= specific heat capacity at constant pressure = 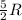 (For diatomic gas)
(For diatomic gas)