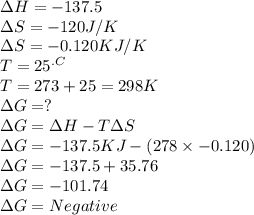Chemistry, 19.07.2019 22:20 haleybain6353
C2h4(g) + h2(g) → c2h6(g) δh = –137.5 kj; δs = –120.5 j/k calculate δg at 25 °c and determine whether the reaction is spontaneous. does δg become more negative or more positive as the temperature increases?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
C2h4(g) + h2(g) → c2h6(g) δh = –137.5 kj; δs = –120.5 j/k calculate δg at 25 °c and determine wheth...
Questions







Computers and Technology, 29.10.2019 04:31

Mathematics, 29.10.2019 04:31