Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
You know the right answer?
Calculate the change in entropy that occurs in the system when 4.20 mole of diethyl ether (\(\rm c_4...
Questions

History, 25.01.2020 20:31

Mathematics, 25.01.2020 20:31



Spanish, 25.01.2020 20:31

English, 25.01.2020 20:31

Mathematics, 25.01.2020 20:31


Computers and Technology, 25.01.2020 20:31

Mathematics, 25.01.2020 20:31


History, 25.01.2020 20:31

Health, 25.01.2020 20:31


Physics, 25.01.2020 20:31




English, 25.01.2020 20:31

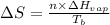
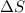 = entropy change of the system = ?
= entropy change of the system = ?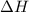 = enthalpy of vaporization = 34.6 kJ/mole
= enthalpy of vaporization = 34.6 kJ/mole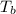 = normal boiling point =
= normal boiling point =