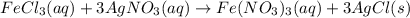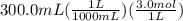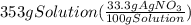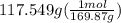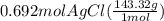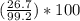Chemistry, 19.07.2019 20:10 laurielaparr2930
300. ml of a 3.0 m aqueous solution of iron (iii) chloride is mixed with 353 grams of a 33.3 mass % solution of silver (u) nitrate and water. 267 grams of a solid precipitate forms. what is the percent yield of the reaction assuming that the solubility of the solid precipitate in water is negligible.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
300. ml of a 3.0 m aqueous solution of iron (iii) chloride is mixed with 353 grams of a 33.3 mass %...
Questions


Mathematics, 22.10.2021 14:00



English, 22.10.2021 14:00

English, 22.10.2021 14:00

Mathematics, 22.10.2021 14:00


Mathematics, 22.10.2021 14:00

History, 22.10.2021 14:00


Mathematics, 22.10.2021 14:00

Chemistry, 22.10.2021 14:00


Mathematics, 22.10.2021 14:00


English, 22.10.2021 14:00


Mathematics, 22.10.2021 14:00

English, 22.10.2021 14:00