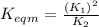Chemistry, 19.07.2019 04:30 yarielisr18
Use the reactions below and their equilibrium constants to predict the equilibrium constant for the reaction 2a(s)⇌3d(g). a(s) ⇌ 12 b(g)+c(g), k1=0.0334 3d(g) ⇌ b(g)+2c(g), k2=2.35

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1
You know the right answer?
Use the reactions below and their equilibrium constants to predict the equilibrium constant for the...
Questions


Mathematics, 26.02.2021 01:20

Mathematics, 26.02.2021 01:20

Social Studies, 26.02.2021 01:20


History, 26.02.2021 01:20

Mathematics, 26.02.2021 01:20

Mathematics, 26.02.2021 01:20

Mathematics, 26.02.2021 01:20


Mathematics, 26.02.2021 01:20


Mathematics, 26.02.2021 01:20


Biology, 26.02.2021 01:20


Mathematics, 26.02.2021 01:20

Geography, 26.02.2021 01:20

Mathematics, 26.02.2021 01:20

Mathematics, 26.02.2021 01:20


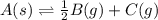
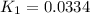


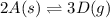

 for the final reaction.
for the final reaction.