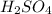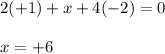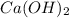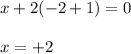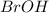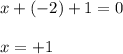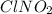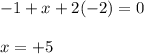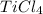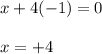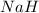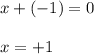Chemistry, 19.07.2019 02:10 Brightcord9679
Determine the oxidation states of the elements in the compounds listed. none of the oxygen-containing compounds are peroxides or superoxides. (a) h2so4 (b) ca(oh)2 (c) broh (d) clno2 (e) ticl4 (f) nah'

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3


Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
Determine the oxidation states of the elements in the compounds listed. none of the oxygen-containin...
Questions

Health, 24.09.2019 10:30

Biology, 24.09.2019 10:30

Biology, 24.09.2019 10:30


Physics, 24.09.2019 10:30

Mathematics, 24.09.2019 10:30

Mathematics, 24.09.2019 10:30

Biology, 24.09.2019 10:30



Social Studies, 24.09.2019 10:30



History, 24.09.2019 10:30

Mathematics, 24.09.2019 10:30



History, 24.09.2019 10:30

Mathematics, 24.09.2019 10:30