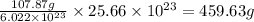Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3


Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
Determine how many grams of silver would be produced, if 12.83 x 10^23 atoms of copper react with an...
Questions

Physics, 19.08.2020 18:01


Mathematics, 19.08.2020 18:01


Mathematics, 19.08.2020 18:01

Health, 19.08.2020 18:01


Mathematics, 19.08.2020 18:01

English, 19.08.2020 18:01

Mathematics, 19.08.2020 18:01


Mathematics, 19.08.2020 18:01


Advanced Placement (AP), 19.08.2020 18:01

History, 19.08.2020 18:01


History, 19.08.2020 18:01

Mathematics, 19.08.2020 18:01


Mathematics, 19.08.2020 18:01

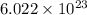 number of atoms.
number of atoms.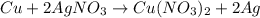
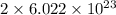 number of atoms of silver.
number of atoms of silver.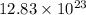 number of atoms of copper will produce =
number of atoms of copper will produce = 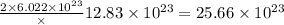 number of atoms of silver.
number of atoms of silver.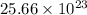 number of atoms will occupy =
number of atoms will occupy =