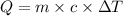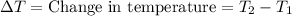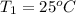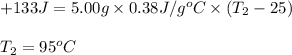Chemistry, 19.07.2019 01:20 katelynn73
A5.00-g sample of copper metal at 25.0 °c is heated by the addition of 133 j of energy. the final temperature of the copper is °c. the specific heat capacity of copper is 0.38 j/g°c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
A5.00-g sample of copper metal at 25.0 °c is heated by the addition of 133 j of energy. the final te...
Questions

Mathematics, 29.10.2020 07:30

Geography, 29.10.2020 07:30

History, 29.10.2020 07:30

Social Studies, 29.10.2020 07:30


Arts, 29.10.2020 07:30

History, 29.10.2020 07:30


Mathematics, 29.10.2020 07:30

Chemistry, 29.10.2020 07:30

Biology, 29.10.2020 07:30

Health, 29.10.2020 07:30


Mathematics, 29.10.2020 07:30

Social Studies, 29.10.2020 07:30




Mathematics, 29.10.2020 07:30

Mathematics, 29.10.2020 07:30