
Chemistry, 18.07.2019 23:30 kalialee2424
How many grams of nahco3 (fm 84.01 g/mol) should be mixed with na2co3 to produce a 1.00 l buffer solution with ph 9.50. the final concentration of na2co3 in this solution is 0.10 m. pka1 = 6.37 and pka2 = 10.33 for h2co3.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
__ _ _ _ _ is the process of removing earth materials from their original sites through weathering and transport and depositing the in another location. a. erosion b. sedimentation c. lithification d. dissolution
Answers: 1

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1
You know the right answer?
How many grams of nahco3 (fm 84.01 g/mol) should be mixed with na2co3 to produce a 1.00 l buffer sol...
Questions

Mathematics, 11.06.2021 19:50


Geography, 11.06.2021 19:50

Mathematics, 11.06.2021 19:50




Mathematics, 11.06.2021 19:50



Mathematics, 11.06.2021 19:50

Health, 11.06.2021 19:50


Geography, 11.06.2021 19:50



History, 11.06.2021 19:50


Physics, 11.06.2021 19:50

Biology, 11.06.2021 19:50

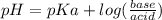
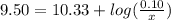
 is the concentration of sodium bicarbonate)
is the concentration of sodium bicarbonate)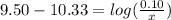
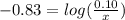
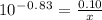
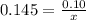
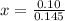
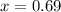
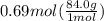
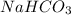 will be required.
will be required. 

