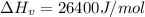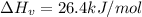Chemistry, 18.07.2019 22:30 crybaby222psyc
The estimated heat of vaporization of diethyl ether using the chen's rule is a. 29.7 kj/mol b. 33.5 kj/mol c. 26.4 kj/mol d. 36.8 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1


Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1
You know the right answer?
The estimated heat of vaporization of diethyl ether using the chen's rule is a. 29.7 kj/mol b. 33.5...
Questions

Chemistry, 09.11.2020 21:20

World Languages, 09.11.2020 21:20




English, 09.11.2020 21:20

Mathematics, 09.11.2020 21:20

Mathematics, 09.11.2020 21:20

Social Studies, 09.11.2020 21:20


Social Studies, 09.11.2020 21:20

Arts, 09.11.2020 21:20

Mathematics, 09.11.2020 21:20

Biology, 09.11.2020 21:20

Mathematics, 09.11.2020 21:20

Computers and Technology, 09.11.2020 21:20




![\Delta H_v=RT_b\left [ \frac{3.974\left ( \frac{T_b}{T_c} \right )-3.958+1.555lnP_c}{1.07-\left ( \frac{T_b}{T_c} \right )} \right ]](/tpl/images/0105/5856/32a5b.png)
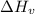 is the Heat of vaoprization (J/mol)
is the Heat of vaoprization (J/mol)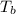 is the normal boiling point of the gas (K)
is the normal boiling point of the gas (K)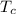 is the Critical temperature of the gas (K)
is the Critical temperature of the gas (K)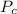 is the Critical pressure of the gas (bar)
is the Critical pressure of the gas (bar)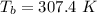
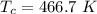
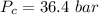
![\Delta H_v=8.314\times307.4 \left [ \frac{3.974\left ( \frac{307.4}{466.7} \right )-3.958+1.555ln36.4}{1.07-\left ( \frac{307.4}{466.7} \right )} \right ]](/tpl/images/0105/5856/04a03.png)