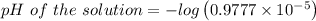Chemistry, 18.07.2019 20:30 loveniasummer71
Ammonium chloride, nh4cl, is a salt formed from the neutralization of the weak base ammonia, nh3, with the strong acid hydrochloric acid. given that the value of kb for ammonia is 1.8×10−5, what is the ph of a 0.176 m solution of ammonium chloride at 25∘c?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

You know the right answer?
Ammonium chloride, nh4cl, is a salt formed from the neutralization of the weak base ammonia, nh3, wi...
Questions




History, 05.05.2020 19:46

Mathematics, 05.05.2020 19:46



Biology, 05.05.2020 19:46


Physics, 05.05.2020 19:57

Biology, 05.05.2020 19:57

Social Studies, 05.05.2020 19:57


Mathematics, 05.05.2020 19:57





Biology, 05.05.2020 19:57

![\left[H^+ \right]=\sqrt{K_a\times C}](/tpl/images/0105/2520/72ad6.png)
![\left[H^+ \right]=\sqrt{5.5556\times 10^{-10}\times 0.176}](/tpl/images/0105/2520/b4744.png)
![\left[H^+ \right]=0.9777\times 10^{-5}](/tpl/images/0105/2520/5c7e7.png)
![pH\ of\ the\ solution=-log\left[H^+ \right]](/tpl/images/0105/2520/ec689.png)