
Chemistry, 16.07.2019 12:10 kinziemadison12
Asample of cesium carbonate, weighing 3.80 g, requires 1.90 g of hydrogen bromide gas to completely decompose to water, cesium bromide, and carbon dioxide gas. the total mass of water and cesium bromide formed is 5.20 g and no hydrogen bromide or cesium carbonate remains. according to the law of conservation of mass, what mass of carbon dioxide must have been formed?
a. 0.50 g
b 1.40 g
c 5.49 g
d 10.90 g
e 1.90 g
give me an explanation on why it is the correct answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2


Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
Asample of cesium carbonate, weighing 3.80 g, requires 1.90 g of hydrogen bromide gas to completely...
Questions


Mathematics, 04.05.2021 05:30

English, 04.05.2021 05:30




English, 04.05.2021 05:30




Mathematics, 04.05.2021 05:30


Mathematics, 04.05.2021 05:30

Mathematics, 04.05.2021 05:30

Chemistry, 04.05.2021 05:30


Health, 04.05.2021 05:30


Mathematics, 04.05.2021 05:30

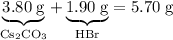 .
.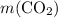 represent the mass of carbon dioxide produced in this reaction.
represent the mass of carbon dioxide produced in this reaction.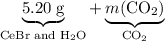 .
.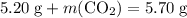 .
.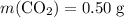 .
.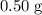 .
.


