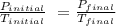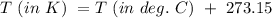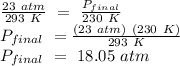Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
A5 l tank of oxygen with a pressure of 23 atm is moved from room temperature of 293 k to a storage f...
Questions


English, 01.09.2019 10:50



Mathematics, 01.09.2019 10:50




Health, 01.09.2019 10:50

Social Studies, 01.09.2019 10:50


Mathematics, 01.09.2019 10:50

History, 01.09.2019 10:50


Mathematics, 01.09.2019 10:50



Biology, 01.09.2019 10:50

Social Studies, 01.09.2019 10:50

History, 01.09.2019 10:50