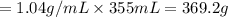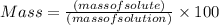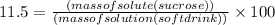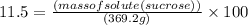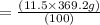Chemistry, 15.07.2019 06:10 karinagaticap73vrn
Asoft drink contains 11.5% sucrose (c12h22o11) by mass. how much sucrose, in grams, is contained in 355 ml (12 oz) of the soft drink? (assume a density of 1.04 g/ml.)
express your answer to three significant figures and include appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
Asoft drink contains 11.5% sucrose (c12h22o11) by mass. how much sucrose, in grams, is contained in...
Questions

Biology, 27.11.2020 23:50


Advanced Placement (AP), 27.11.2020 23:50





History, 27.11.2020 23:50


English, 27.11.2020 23:50


Mathematics, 27.11.2020 23:50

Mathematics, 27.11.2020 23:50


Mathematics, 27.11.2020 23:50