Chemistry, 13.07.2019 23:20 roxymiller3942
The standard cell potential ec for the reduction of silver ions with elemental copper is 0.46v at 25 degrees celsius. calculate δg for this reaction.
*** explain the reactions since i’m very confused as to wich side i should put the electrons.
ex: cu-> cu2+ + 2e

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1

Chemistry, 23.06.2019 08:00
Problem page a jet airplane reaches 846. km/h on a certain flight. what distance does it cover in 13.0 min? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.
Answers: 2
You know the right answer?
The standard cell potential ec for the reduction of silver ions with elemental copper is 0.46v at 25...
Questions

Biology, 22.09.2019 05:50

Health, 22.09.2019 05:50


English, 22.09.2019 05:50


History, 22.09.2019 05:50


Mathematics, 22.09.2019 05:50



Biology, 22.09.2019 05:50

Computers and Technology, 22.09.2019 05:50

History, 22.09.2019 05:50


English, 22.09.2019 05:50

Mathematics, 22.09.2019 05:50

Computers and Technology, 22.09.2019 05:50

Mathematics, 22.09.2019 06:00

Mathematics, 22.09.2019 06:00

Biology, 22.09.2019 06:00

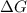 for this reaction is, -88780 J/mole.
for this reaction is, -88780 J/mole.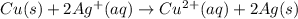
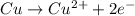
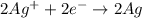
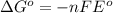
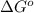 = Gibbs free energy = ?
= Gibbs free energy = ?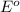 = standard e.m.f of cell = 0.46 V
= standard e.m.f of cell = 0.46 V