
Chemistry, 13.07.2019 05:20 thegamingkid914
Classify each of these soluble solutes as a strong electrolyte, a weak electrolyte, or a nonelectrolyte. solutes formula nitric acid hno3 calcium hydroxide ca(oh)2 acetic acid h3ccooh methyl amine ch3nh2 potassium chloride kcl ethanol c2h5oh glucose c6h12o6

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2
You know the right answer?
Classify each of these soluble solutes as a strong electrolyte, a weak electrolyte, or a nonelectrol...
Questions









Physics, 21.01.2021 14:00

History, 21.01.2021 14:00






Physics, 21.01.2021 14:00

Biology, 21.01.2021 14:00

Mathematics, 21.01.2021 14:00

Mathematics, 21.01.2021 14:00

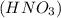 Nitric acid,
Nitric acid, 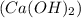 )Calcium Hydroxide and (KCl) potassium chloride
)Calcium Hydroxide and (KCl) potassium chloride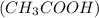 Acetic acid and
Acetic acid and 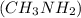 Methyl amine
Methyl amine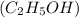 Ethanol and
Ethanol and 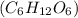 Glucose
Glucose 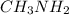 Methyl amine are partially ionized when dissolved in polar solvent so these electrolytes are weak electrolytes.
Methyl amine are partially ionized when dissolved in polar solvent so these electrolytes are weak electrolytes.


