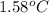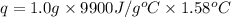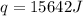Chemistry, 13.07.2019 01:30 jmanrules200
When 1.0 g of fructose, c6h12o6(s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the calorimeter increases by 1.58 °c. if the heat capacity of the calorimeter and its contents is 9.90 kj/°c, what is q for this combustion?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1


Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
When 1.0 g of fructose, c6h12o6(s), a sugar commonly found in fruits, is burned in oxygen in a bomb...
Questions










History, 10.11.2020 17:00









Physics, 10.11.2020 17:00

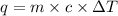
 = heat of combustion = ?
= heat of combustion = ?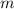 = mass of fructose = 1.0 g
= mass of fructose = 1.0 g = heat capacity of the calorimteter =
= heat capacity of the calorimteter = 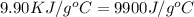
 = change in temperature =
= change in temperature =