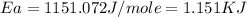Chemistry, 13.07.2019 00:20 kashusledbetter
Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollution generated in automobile engines. the decomposition of nitric oxide (no) to n2 and o2 is second order with a rate constant of 0.0796 m−1⋅s−1 at 737∘c and 0.0815 m−1⋅s−1 at 947∘c. calculate the activation energy for the reaction in kj/mol

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1


Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollutio...
Questions




Mathematics, 19.02.2020 01:47




History, 19.02.2020 01:47









Mathematics, 19.02.2020 01:47


Mathematics, 19.02.2020 01:47

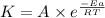
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0082/8135/6d953.png)
 = rate constant at
= rate constant at 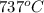 =
= 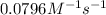
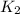 = rate constant at
= rate constant at 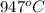 =
= 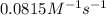
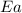 = activation energy for the reaction = ?
= activation energy for the reaction = ?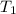 = initial temperature =
= initial temperature = 
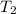 = final temperature =
= final temperature = 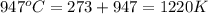
![\log (\frac{0.0815M^{-1}s^{-1}}{0.0796M^{-1}s^{-1}})=\frac{Ea}{2.303\times 8.314J/mole.K}[\frac{1}{1010K}-\frac{1}{1220K}]](/tpl/images/0082/8135/b28e1.png)