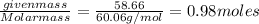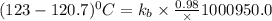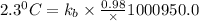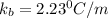Chemistry, 12.07.2019 20:30 joannegrace869
The normal boiling point of a certain liquid x is 120.7°c , but when 58.66g of urea nh22co are dissolved in 950.g of x , it is found that the solution boils at 123.0°c instead. use this information to calculate the molal boiling point elevation constant kb of x .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
The normal boiling point of a certain liquid x is 120.7°c , but when 58.66g of urea nh22co are disso...
Questions

Mathematics, 05.01.2020 19:31

Mathematics, 05.01.2020 19:31


Biology, 05.01.2020 19:31

Mathematics, 05.01.2020 19:31


French, 05.01.2020 19:31


Social Studies, 05.01.2020 19:31


Mathematics, 05.01.2020 19:31


Mathematics, 05.01.2020 19:31

History, 05.01.2020 19:31

Chemistry, 05.01.2020 19:31

Mathematics, 05.01.2020 19:31

Mathematics, 05.01.2020 19:31



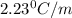
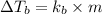
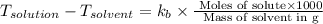
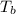 = change in boiling point
= change in boiling point
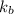 = boiling point constant
= boiling point constant