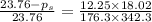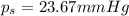The vapor pressure of water is 23.76 mm hg at 25 °c. a nonvolatile, nonelectrolyte that dissolves in water is sucrose. calculate the vapor pressure of the solution at 25 °c when 12.25 grams of sucrose, c12h22o11 (342.3 g/mol), are dissolved in 176.3 grams of water. water = h2o = 18.02 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 23.06.2019 02:00
Which would freeze at a higher temperature: the great salt lake or lake tahoe? a. lake tahoe would freeze at a higher temperature. b. the great salt lake would freeze at a higher temperature. c. both lakes would freeze at the same temperature.
Answers: 2

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1
You know the right answer?
The vapor pressure of water is 23.76 mm hg at 25 °c. a nonvolatile, nonelectrolyte that dissolves in...
Questions

Chemistry, 17.07.2019 17:30

Social Studies, 17.07.2019 17:30



Spanish, 17.07.2019 17:30


Chemistry, 17.07.2019 17:30

Mathematics, 17.07.2019 17:30

Mathematics, 17.07.2019 17:30




Mathematics, 17.07.2019 17:30

History, 17.07.2019 17:30

Social Studies, 17.07.2019 17:30

History, 17.07.2019 17:30

Social Studies, 17.07.2019 17:30

Social Studies, 17.07.2019 17:30

Social Studies, 17.07.2019 17:30

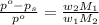
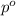 = vapor pressure of pure solvent (water) = 23.76 mmHg
= vapor pressure of pure solvent (water) = 23.76 mmHg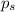 = vapor pressure of solution= ?
= vapor pressure of solution= ?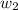 = mass of solute (sucrose) = 12.25 g
= mass of solute (sucrose) = 12.25 g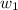 = mass of solvent (water) = 176.3 g
= mass of solvent (water) = 176.3 g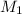 = molar mass of solvent (water) = 18.02 g/mole
= molar mass of solvent (water) = 18.02 g/mole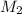 = molar mass of solute (sucrose) = 342.3 g/mole
= molar mass of solute (sucrose) = 342.3 g/mole