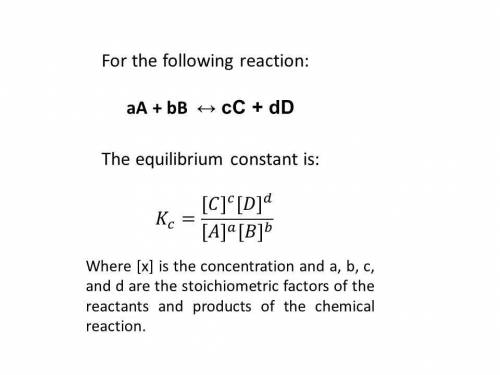
Consider the following reaction and its equilibrium constant: 4 cuo(s) + ch4(g) ⇌ co2(g) + 4 cu(s) + 2 h2o(g) kc = 1.10.
a reaction mixture contains 0.22 m ch4, 0.70 m co2 and 1.5 m h2o. which of the following statements is true concerning this system? a. the reaction will shift in the direction of products. b. the equilibrium constant will increase. c. the reaction will shift in the direction of reactants. d. the system is at equilibrium.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which type of stress results when two plates push against one another? a. compression b. tension c. force d. shear
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
Consider the following reaction and its equilibrium constant: 4 cuo(s) + ch4(g) ⇌ co2(g) + 4 cu(s)...
Questions

Spanish, 11.11.2020 21:50

Mathematics, 11.11.2020 21:50

English, 11.11.2020 21:50

Mathematics, 11.11.2020 21:50


History, 11.11.2020 21:50






Social Studies, 11.11.2020 21:50

Mathematics, 11.11.2020 21:50


Mathematics, 11.11.2020 21:50


Mathematics, 11.11.2020 21:50

History, 11.11.2020 21:50


History, 11.11.2020 21:50

![Q = \frac{[CO_{2}][H_{2}O]^{2}}{[CH_{4}]}](/tpl/images/0072/0904/ac4b6.png) (2)
(2)![Q = \frac{[CO_{2}][H_{2}O]^{2}}{[CH_{4}]} = \frac{0.70*(1.5)^{2}}{0.22} = 7.16](/tpl/images/0072/0904/5a31b.png)