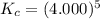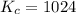Chemistry, 09.07.2019 03:20 chrismcb875
Determine the value of kc for the following reaction if the equilibrium concentrations are as follows: [p4o10]eq = 2.000 moles, [p4]eq = 3.000 moles, [o2]eq = 4.000 m p4o10(s) ↔ p4(s) + 5 o2(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
You know the right answer?
Determine the value of kc for the following reaction if the equilibrium concentrations are as follow...
Questions

History, 14.10.2020 16:01

Mathematics, 14.10.2020 16:01

History, 14.10.2020 16:01

Social Studies, 14.10.2020 16:01

Mathematics, 14.10.2020 16:01

Biology, 14.10.2020 16:01

History, 14.10.2020 16:01


Mathematics, 14.10.2020 16:01

Mathematics, 14.10.2020 16:01

Business, 14.10.2020 16:01

Biology, 14.10.2020 16:01





Biology, 14.10.2020 16:01

Mathematics, 14.10.2020 16:01

Mathematics, 14.10.2020 16:01

Computers and Technology, 14.10.2020 16:01

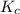 for the following reaction will be, 1024
for the following reaction will be, 1024
![K_c=[O_2]^5](/tpl/images/0067/9324/32ee9.png)