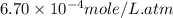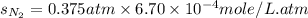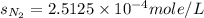Chemistry, 09.07.2019 01:30 glydelxc2780
Calculate the solubility of nitrogen in water at an atmospheric pressure of 0.480 atm (a typical value at high altitude). atmospheric gas mole fraction kh mol/(l*atm) n2 7.81 x 10-1 6.70 x 10-4 o2 2.10 x 10-1 1.30 x 10-3 ar 9.34 x 10-3 1.40 x 10-3 co2 3.33 x 10-4 3.50 x 10-2 ch4 2.00 x 10-6 1.40 x 10-3 h2 5.00 x 10-7 7.80 x 10-4

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
Calculate the solubility of nitrogen in water at an atmospheric pressure of 0.480 atm (a typical val...
Questions


Social Studies, 29.04.2021 06:30


Mathematics, 29.04.2021 06:30

Social Studies, 29.04.2021 06:30

Mathematics, 29.04.2021 06:40





Physics, 29.04.2021 06:40

English, 29.04.2021 06:40



Biology, 29.04.2021 06:40



Physics, 29.04.2021 06:40


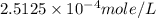
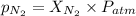
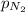 = partial pressure of nitrogen = ?
= partial pressure of nitrogen = ?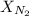 = mole fraction of nitrogen =
= mole fraction of nitrogen = 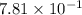
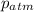 = atmospheric pressure = 0.480 atm
= atmospheric pressure = 0.480 atm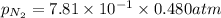
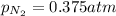
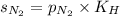
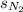 = solubility of nitrogen in water = ?
= solubility of nitrogen in water = ?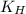 = Henry's constant =
= Henry's constant =