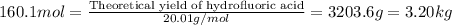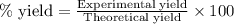Chemistry, 09.07.2019 00:50 robertschulte116
Hydrogen fluoride is used in the manufacture of freons (which destroy ozone in the stratosphere) and in the production of aluminum metal. it is prepared by the reaction caf2 + h2so4 → caso4 + 2hf in one process, 6.25 kg of caf2 is treated with an excess of h2so4 and yields 2.35 kg of hf. calculate the percent yield of hf. % yield

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
Hydrogen fluoride is used in the manufacture of freons (which destroy ozone in the stratosphere) and...
Questions

History, 27.06.2019 11:30

Mathematics, 27.06.2019 11:30

History, 27.06.2019 11:30

English, 27.06.2019 11:30



Geography, 27.06.2019 11:30

Chemistry, 27.06.2019 11:30


Geography, 27.06.2019 11:30








Mathematics, 27.06.2019 11:30

Mathematics, 27.06.2019 11:30

Computers and Technology, 27.06.2019 11:30

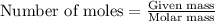 ....(1)
....(1) 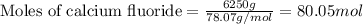
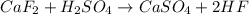
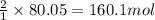 of hydrofluoric acid
of hydrofluoric acid