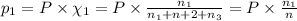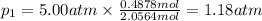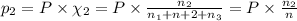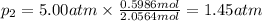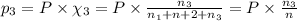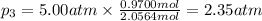Chemistry, 08.07.2019 21:10 cschellfamily
Three gases (8.00 g of methane, ch4, 18.0 g of ethane, c2h6, and an unknown amount of propane, c3h8) were added to the same 10.0-l container. at 23.0 ∘c, the total pressure in the container is 5.00 atm . calculate the partial pressure of each gas in the container.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
You know the right answer?
Three gases (8.00 g of methane, ch4, 18.0 g of ethane, c2h6, and an unknown amount of propane, c3h8)...
Questions


Mathematics, 05.10.2019 10:30

History, 05.10.2019 10:30

Mathematics, 05.10.2019 10:30

Physics, 05.10.2019 10:30

English, 05.10.2019 10:30


Mathematics, 05.10.2019 10:30

Mathematics, 05.10.2019 10:30







Geography, 05.10.2019 10:30

History, 05.10.2019 10:30



Biology, 05.10.2019 10:40

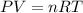 (Ideal gas equation)
(Ideal gas equation)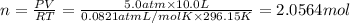
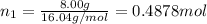
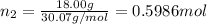
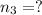
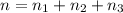
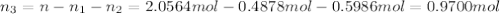
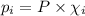
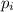 = partial pressure of 'i' component.
= partial pressure of 'i' component.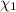 = mole fraction of 'i' component in mixture
= mole fraction of 'i' component in mixture