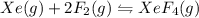Chemistry, 08.07.2019 18:10 alemorachis49
1) consider the following reaction at equilibrium. what effect will reducing the pressure of the reaction mixture have on the system? xe(g) + 2 f2(g) ? xef4(g) a)the equilibrium constant will decrease. b)no effect will be observed. c)the reaction will shift to the right in the direction of products. d) the equilibrium constant will increase e) the reaction will shift to the left in the direction of reactants.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
1) consider the following reaction at equilibrium. what effect will reducing the pressure of the rea...
Questions





Physics, 20.08.2021 19:30




English, 20.08.2021 19:40

Social Studies, 20.08.2021 19:40

Chemistry, 20.08.2021 19:40





Mathematics, 20.08.2021 19:40

Mathematics, 20.08.2021 19:40