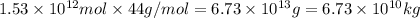Calcium oxide or quicklime (cao) is used in steelmaking, cement manufacture, and pollution control. it is prepared by the thermal decomposition of calcium carbonate: caco3(s) → cao(s) co2(g) calculate the yearly release of co2 (in kg) to the atmosphere if the annual production of cao in the united states is 8.6 × 1010 kg.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3


Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

You know the right answer?
Calcium oxide or quicklime (cao) is used in steelmaking, cement manufacture, and pollution control....
Questions


Social Studies, 29.09.2020 09:01




History, 29.09.2020 09:01






Mathematics, 29.09.2020 09:01


Mathematics, 29.09.2020 09:01




Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01

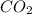 into the atmosphere is
into the atmosphere is 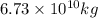 .
.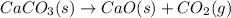
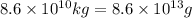
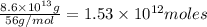
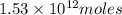 of CaO moles of carbon-dioxide moles produced will be:
of CaO moles of carbon-dioxide moles produced will be: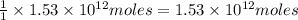 of carbon-dioxide
of carbon-dioxide moles of carbon-dioxide:
moles of carbon-dioxide: