
Chemistry, 05.02.2020 12:00 hillisaiah734
The reform reaction between steam and gaseous methane (ch4) produces "synthesis gas," a mixture of carbon monoxide gas and dihydrogen gas. synthesis gas is one of the most widely used industrial chemicals, and is the major industrial source of hydrogen.
suppose a chemical engineer studying a new catalyst for the reform reaction finds that 924. liters per second of methane are consumed when the reaction is run at 261.°c and 0.96atm. calculate the rate at which dihydrogen is being produced. give your answer in kilograms per second. round your answer to 2 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3


Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
The reform reaction between steam and gaseous methane (ch4) produces "synthesis gas," a mixture of c...
Questions

Social Studies, 15.12.2020 18:50

Health, 15.12.2020 18:50





Physics, 15.12.2020 18:50

Mathematics, 15.12.2020 18:50


Biology, 15.12.2020 18:50

Mathematics, 15.12.2020 18:50

English, 15.12.2020 18:50




Mathematics, 15.12.2020 18:50

Mathematics, 15.12.2020 18:50



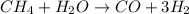 Haber reaction
Haber reaction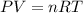
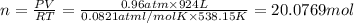
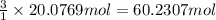 of dihydrogen
of dihydrogen

