
Chemistry, 08.07.2019 17:30 meramera50
Be sure to answer all parts. propane (c3h8) is a minor component of natural gas and is used in domestic cooking and heating. (a) balance the following equation representing the combustion of propane in air. include states of matter in your answer. c3h8(g) + o2(g) → co2(g) + h2o(g) (b) how many grams of carbon dioxide can be produced by burning 8.11 moles of propane? assume that oxygen is the excess reactant in this reaction. × 10 g enter your answer in scientific notation.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:20
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1
You know the right answer?
Be sure to answer all parts. propane (c3h8) is a minor component of natural gas and is used in domes...
Questions

Mathematics, 23.08.2019 09:50

English, 23.08.2019 09:50

Mathematics, 23.08.2019 09:50

Physics, 23.08.2019 09:50


Mathematics, 23.08.2019 09:50

History, 23.08.2019 09:50


English, 23.08.2019 09:50

History, 23.08.2019 09:50

Chemistry, 23.08.2019 09:50

Social Studies, 23.08.2019 09:50

History, 23.08.2019 09:50


English, 23.08.2019 09:50

English, 23.08.2019 09:50




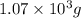
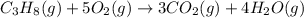
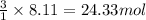 of carbon dioxide gas.
of carbon dioxide gas.
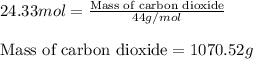
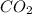 produced in the given reaction and expressed in scientific notation is
produced in the given reaction and expressed in scientific notation is 

