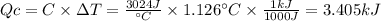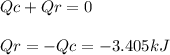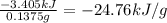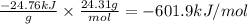Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2

You know the right answer?
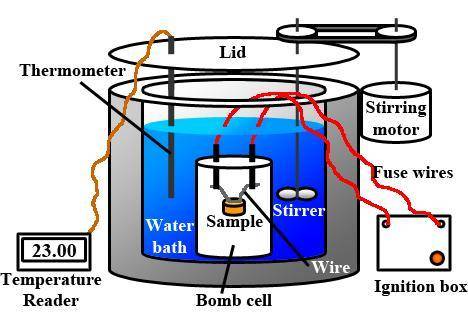
A0.1375-g sample of solid magnesium is burned in a constant-volume bomb calorimeter that has a heat...
Questions

Mathematics, 28.10.2019 17:31

History, 28.10.2019 17:31






Physics, 28.10.2019 17:31

Mathematics, 28.10.2019 17:31


History, 28.10.2019 17:31

Spanish, 28.10.2019 17:31





Mathematics, 28.10.2019 17:31

Arts, 28.10.2019 17:31