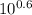Chemistry, 30.01.2020 02:03 lewisf2578
Calculation of original ph from final ph after titration a biochemist has 100 ml of a 0.10 m solution of a weak acid with a pka of 6.3. she adds 6.0 ml of 1.0 m hcl, which changes the ph to 5.7. what was the ph of the original solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
Calculation of original ph from final ph after titration a biochemist has 100 ml of a 0.10 m solutio...
Questions

English, 27.10.2020 19:30


Mathematics, 27.10.2020 19:30

Engineering, 27.10.2020 19:30

Mathematics, 27.10.2020 19:30


Mathematics, 27.10.2020 19:30

Advanced Placement (AP), 27.10.2020 19:30


Physics, 27.10.2020 19:30









Biology, 27.10.2020 19:30

History, 27.10.2020 19:30