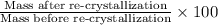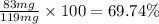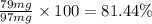Chemistry, 06.07.2019 03:20 savannahvargas512
Amixture of two compounds, a and b, was separated by extraction. after the compounds were dried, their masses were found to be: 119 mg of compound a and 97 mg of compound b. both compounds were recrystallized and weighed again. after recrystallization, the mass of compound a was 83 mg and the mass of compound b was 79 mg. calculate the percent recovery from recrystallization for both compounds.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
Amixture of two compounds, a and b, was separated by extraction. after the compounds were dried, the...
Questions



Health, 16.10.2019 17:30

English, 16.10.2019 17:30



Physics, 16.10.2019 17:30




Mathematics, 16.10.2019 17:30


Mathematics, 16.10.2019 17:30



World Languages, 16.10.2019 17:30

English, 16.10.2019 17:30

Biology, 16.10.2019 17:30


English, 16.10.2019 17:30