
Chemistry, 06.07.2019 03:20 yazmineespinozarive
Determine the amount of heat (in kj) given off when 1.26 × 104 g of ammonia are produced according to the equation n2(g) + 3h2(g) ⟶ 2nh3(g) δh°rxn = −92.6 kj/mol assume that the reaction takes place under standardstate conditions at 25°c.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1
You know the right answer?
Determine the amount of heat (in kj) given off when 1.26 × 104 g of ammonia are produced according t...
Questions


Chemistry, 20.04.2020 05:18


Computers and Technology, 20.04.2020 05:18


Mathematics, 20.04.2020 05:18



Computers and Technology, 20.04.2020 05:19


Mathematics, 20.04.2020 05:19


Mathematics, 20.04.2020 05:19




Mathematics, 20.04.2020 05:19

Mathematics, 20.04.2020 05:19

Mathematics, 20.04.2020 05:19

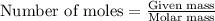
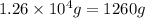
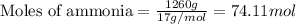
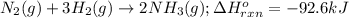
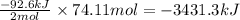 of energy.
of energy.

