
Chemistry, 06.07.2019 01:20 blackwelle72
Which statements are true? δ for an exothermic reaction is positive. δ for an endothermic reaction is positive. the evaporation of water is an exothermic process. a combustion reaction is exothermic. when energy is transferred as heat from the surroundings to the system, δ is negative. when energy is transferred as heat from the system to the surroundings, δ is negative.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1


Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
Which statements are true? δ for an exothermic reaction is positive. δ for an endothermic reaction...
Questions

Mathematics, 30.05.2020 13:57


Mathematics, 30.05.2020 13:57

Biology, 30.05.2020 13:57


Mathematics, 30.05.2020 13:57




Chemistry, 30.05.2020 13:57

Biology, 30.05.2020 13:57

Mathematics, 30.05.2020 13:57





Mathematics, 30.05.2020 13:57


English, 30.05.2020 13:57

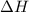 for an endothermic reaction is positive, a combustion reaction is exothermic and when energy is transferred as heat from the system to the surroundings,
for an endothermic reaction is positive, a combustion reaction is exothermic and when energy is transferred as heat from the system to the surroundings, 

