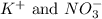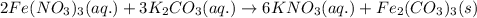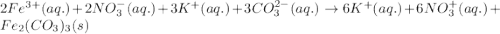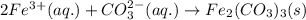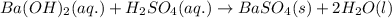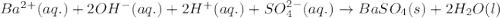Chemistry, 06.07.2019 00:30 danielle413
When the following two solutions are mixed:
k2co3(aq)+fe(no3)3(aq)
the mixture contains the ions listed below. sort these species into spectator ions and ions that react.
drag the appropriate items to their respective bins.
no3-)aq), fe3+ , co3 2-, k+
part b
what is the correct net ionic equation, including all coefficients, charges, and phases, for the following set of reactants? assume that the contribution of protons from h2so4 is near 100 %.
ba(oh)2(aq)+h2so4(aq)?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

You know the right answer?
When the following two solutions are mixed:
k2co3(aq)+fe(no3)3(aq)
the mixture contains...
k2co3(aq)+fe(no3)3(aq)
the mixture contains...
Questions


Mathematics, 06.03.2021 07:00


Mathematics, 06.03.2021 07:00

Biology, 06.03.2021 07:00

Mathematics, 06.03.2021 07:00

Social Studies, 06.03.2021 07:00


English, 06.03.2021 07:00





Mathematics, 06.03.2021 07:00






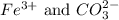 and the ions that react are
and the ions that react are