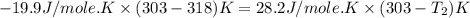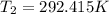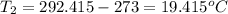Chemistry, 05.07.2019 22:30 itaheart101
Two systems with heat capacities 19.9 j mol-1 k-1 and 28.2 ] mol 1 k-1 respectively interact thermally and come to an equilibrium temperature of 300c. if the initial temperature of system 1 was 450c, what was the initial temperature of system 2 in °c? you may assume that the total energy of the combined systems remains constant

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1
You know the right answer?
Two systems with heat capacities 19.9 j mol-1 k-1 and 28.2 ] mol 1 k-1 respectively interact thermal...
Questions








Mathematics, 14.12.2020 02:30


Mathematics, 14.12.2020 02:30








Mathematics, 14.12.2020 02:30


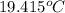
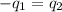
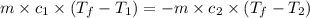
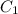 = heat capacity of system 1 = 19.9 J/mole.K
= heat capacity of system 1 = 19.9 J/mole.K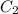 = heat capacity of system 2 = 28.2 J/mole.K
= heat capacity of system 2 = 28.2 J/mole.K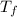 = final temperature of system =
= final temperature of system = 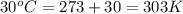
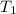 = initial temperature of system 1 =
= initial temperature of system 1 = 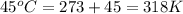
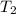 = initial temperature of system 2 = ?
= initial temperature of system 2 = ?