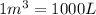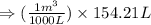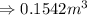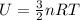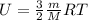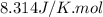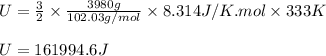Chemistry, 05.07.2019 22:20 jackiecroce1
Arigid vessel contains 3.98 kg of refrigerant-134a at 700 kpa and 60°c. determine the volume of the vessel and the total internal energy. m3 (round to four decimal places) kj (round to one decimal place)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 07:30
Achemist at a pharmaceutical company is measuring equilibrium constants for reactions in which drug candidate molecules bind to a protein involved in cancer. the drug molecules bind the protein in a 1: 1 ratio to form a drug-protein complex. the protein concentration in aqueous solution at 25 ˚c is 1.74 x10-6 m . drug a is introduced into the protein solution at an initial concentration of 2.00 x10-6m. drug b is introduced into a separate, identical protein solution at an initial concentration of 2.00 x10-6m. at equilibrium, the drug a-protein solution has an a-protein complex concentration of 1.00 x10-6m, and the drug b solution has a b-protein complex concentration of 1.40 x10-6m.a. calculate the kc value for the a-protein binding reaction.b. calculate the kc value for the b-protein binding reaction.c. assuming that the drug that binds more strongly will be more effective, which drug is the better choice for further research?
Answers: 1

You know the right answer?
Arigid vessel contains 3.98 kg of refrigerant-134a at 700 kpa and 60°c. determine the volume of the...
Questions


History, 09.12.2020 18:10


Computers and Technology, 09.12.2020 18:10




Mathematics, 09.12.2020 18:10


Mathematics, 09.12.2020 18:10

History, 09.12.2020 18:10


History, 09.12.2020 18:10






Mathematics, 09.12.2020 18:10

Chemistry, 09.12.2020 18:10

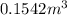 and total internal energy is 162.0 kJ.
and total internal energy is 162.0 kJ. 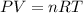
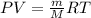
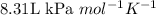
![60^oC=[60+273]K=333K](/tpl/images/0055/6153/72ddf.png)
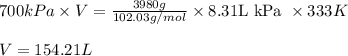
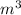 , we use the conversion factor:
, we use the conversion factor: