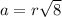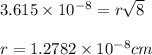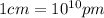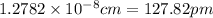Chemistry, 05.07.2019 20:20 bryson9604
Copper crystallizes with a face-centered cubic lattice and has a density of 8.93 g/cm3.
a.) calculate the mass of one unit cell of copper (in grams) b.) calculate the volume of the copper unit cell (in cm3). c.) calculate the edge length of the unit cell (in cm). d.) calculate the radius of a copper atom (in pm).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2
You know the right answer?
Copper crystallizes with a face-centered cubic lattice and has a density of 8.93 g/cm3.
a.) ca...
a.) ca...
Questions



History, 20.07.2019 20:30

Mathematics, 20.07.2019 20:30

Biology, 20.07.2019 20:30

English, 20.07.2019 20:30

Mathematics, 20.07.2019 20:30

English, 20.07.2019 20:30

Mathematics, 20.07.2019 20:30

Geography, 20.07.2019 20:30

History, 20.07.2019 20:30


Mathematics, 20.07.2019 20:30




English, 20.07.2019 20:30

Health, 20.07.2019 20:30

Social Studies, 20.07.2019 20:30

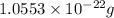
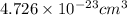
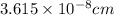
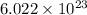 number of atoms.
number of atoms.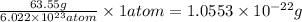
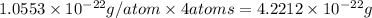
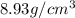
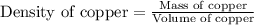
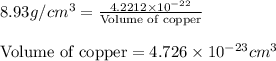
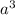
![\sqrt[3]{4.726\times 10^{-23}}cm^3=3.615\times 10^{-8}cm](/tpl/images/0055/3009/53021.png)