
Chemistry, 05.07.2019 20:20 cnejcnefjvefven47
One mole of hydrogen gas is reacted with oxygen gas to produce water in this reaction:
2 h2 + o2 → 2 h2o
in the lab you actually made 14 grams of water. what is the percent yield?
35.60%
100%
11.11%
77.60%

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
One mole of hydrogen gas is reacted with oxygen gas to produce water in this reaction:
2 h2...
2 h2...
Questions


Biology, 11.07.2019 15:00



Biology, 11.07.2019 15:00


Social Studies, 11.07.2019 15:00


Computers and Technology, 11.07.2019 15:00

History, 11.07.2019 15:00








Chemistry, 11.07.2019 15:00


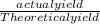 x 100
x 100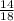 x 100 = 77.6%
x 100 = 77.6%

