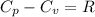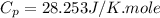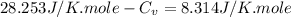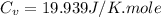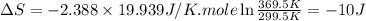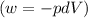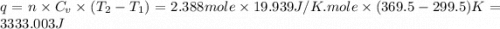Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 2

Chemistry, 23.06.2019 10:00
Abike ride event is 30 miles. a first aid tent is put at the 3/4 mark of the course. how many miles from the starting point is the first aid tent?
Answers: 1
You know the right answer?
Ideal gas (n 2.388 moles) is heated at constant volume from t1 299.5 k to final temperature t2 369.5...
Questions

Mathematics, 10.03.2021 19:30

English, 10.03.2021 19:30

Mathematics, 10.03.2021 19:30

World Languages, 10.03.2021 19:30

Mathematics, 10.03.2021 19:30

Mathematics, 10.03.2021 19:30




Mathematics, 10.03.2021 19:30


Mathematics, 10.03.2021 19:30

Mathematics, 10.03.2021 19:30


Chemistry, 10.03.2021 19:30


Mathematics, 10.03.2021 19:30



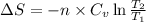
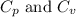 for an ideal gas are :
for an ideal gas are :