

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3


Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1

Chemistry, 23.06.2019 07:00
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table?
Answers: 2
You know the right answer?
The standard enthalpies of formation of so2 and so3 are ‐297 and ‐396 kj/mol respectively. calculate...
Questions

Biology, 07.05.2021 23:50



Computers and Technology, 07.05.2021 23:50



Mathematics, 07.05.2021 23:50

Mathematics, 07.05.2021 23:50

History, 07.05.2021 23:50

Mathematics, 07.05.2021 23:50



English, 07.05.2021 23:50




Medicine, 07.05.2021 23:50



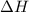
![\Delta H_{rxn}=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0055/1349/db29b.png)
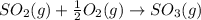
![\Delta H_{rxn}=[(1\times \Delta H_f_{(SO_3)})]-[(1\times \Delta H_f_{(SO_2)})+(\frac{1}{2}\times \Delta H_f_{(O_2)})]](/tpl/images/0055/1349/038bc.png)
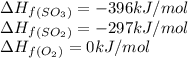
![\Delta H_{rxn}=[(1\times (-396))]-[(1\times (-297))+(\frac{1}{2}\times (0))]\\\\\Delta H_{rxn}=-99kJ](/tpl/images/0055/1349/8b5c5.png)


