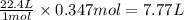Chemistry, 05.07.2019 18:20 manlyman31
What volume (in liters) of carbon monoxide gas, measured at a temperature of 212 k and a pressure of 676 mm hg, is required to synthesize 19.3 g of methanol. how many liters of oxygen (at stp) are required to form 12.5 g of h2o ? show your work

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1
You know the right answer?
What volume (in liters) of carbon monoxide gas, measured at a temperature of 212 k and a pressure of...
Questions


Mathematics, 19.11.2020 22:10

Mathematics, 19.11.2020 22:10



Mathematics, 19.11.2020 22:10


History, 19.11.2020 22:10


History, 19.11.2020 22:10

Mathematics, 19.11.2020 22:10


Social Studies, 19.11.2020 22:10


Arts, 19.11.2020 22:10


Chemistry, 19.11.2020 22:10


Social Studies, 19.11.2020 22:10

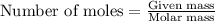 ......(1)
......(1)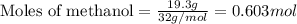
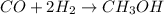
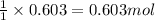 of carbon monoxide.
of carbon monoxide.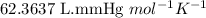
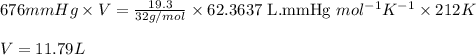
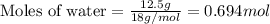
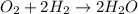
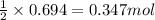 of oxygen gas.
of oxygen gas.