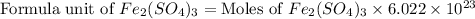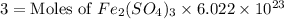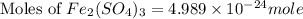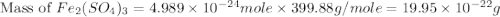The chemical formula for ferric sulfate is fe(so4)3. determine the following:
a) the number of sulfur atoms in 1.75 mole of fe(so4)3
b) the mass in grams of 2.65 mol of fe(so4)3
c) the number of moles of fe(so4)3 in 3.45 grams of fe(so4)3.
d)the mass in grams of 3 formula unit of fe(so4)3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
The chemical formula for ferric sulfate is fe(so4)3. determine the following:
a) the nu...
a) the nu...
Questions

Biology, 21.12.2021 08:40

Mathematics, 21.12.2021 08:40


Health, 21.12.2021 08:40










Advanced Placement (AP), 21.12.2021 08:40




Mathematics, 21.12.2021 08:40


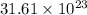 .
.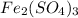 is, 1059.682 grams.
is, 1059.682 grams.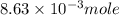
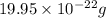
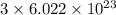 number of sulfur atoms.
number of sulfur atoms.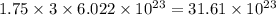 number of sulfur atoms.
number of sulfur atoms.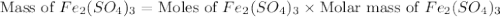
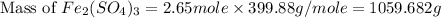
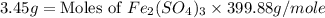
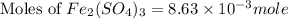
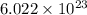 formula unit.
formula unit.