
Chemistry, 05.07.2019 06:10 cupkakekawaii45
Consider the following reaction at equilibrium. what effect will adding more so3 have on the system? so2(g) + no2(g) ⇌ so3(g) + no(g) a) the reaction will shift in the direction of products. b) the reaction will shift to decrease the pressure. c) no change will occur since so3 is not included in the equilibrium expression. d) the reaction will shift in the direction of reactants. e) the equilibrium constant will decrease.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Consider the following reaction at equilibrium. what effect will adding more so3 have on the system?...
Questions

Mathematics, 08.03.2020 04:03




Mathematics, 08.03.2020 04:04

Mathematics, 08.03.2020 04:04


Biology, 08.03.2020 04:05




Mathematics, 08.03.2020 04:06

Advanced Placement (AP), 08.03.2020 04:07


Mathematics, 08.03.2020 04:07

Mathematics, 08.03.2020 04:08




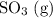 (analog to concentration in a solution.)
(analog to concentration in a solution.) 

