Chemistry, 05.07.2019 05:20 claftonaustin846
Write the balanced chemical equation for the following acid and base reaction. (use the lowest possible whole number coefficients. include states-of-matter under the given conditions in your answer.)
hbr(aq) + lioh(aq) →
a) using the balanced reaction above, calculate the amount of 0.0024 m lioh that would neutralize 22 ml of 0.0026 m hbr.
b)how many moles of salt are produced in the reaction?
c)what is the molar concentration of the salt after the reaction is complete?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 2


You know the right answer?
Write the balanced chemical equation for the following acid and base reaction. (use the lowest possi...
Questions


Mathematics, 09.08.2021 20:40




Mathematics, 09.08.2021 20:40

Mathematics, 09.08.2021 20:40





Computers and Technology, 09.08.2021 20:40

Mathematics, 09.08.2021 20:40






Mathematics, 09.08.2021 20:40

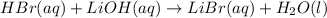
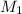 = 0.0024 M
= 0.0024 M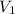
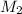 = 0.0026 M
= 0.0026 M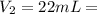
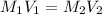
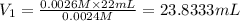
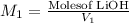
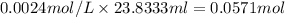
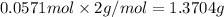
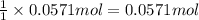 of LiBr
of LiBr