
Chalk is impure calcium carbonate. the amount of calcium carbonate present can be determined by hydrochloric acid to a sample of chalk and measuring the volume of carbon dioxide produced caco3(aq) + 2hcl -> cacl2(aq) + co2(g) + h2o(g) excess hydrochloric acid was added to 0.5g chalk and 100cm3 of carbon dioxide gas was given produced at r. t.p calculate the percentage purity of calcium carbonate in sample of chalk

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

You know the right answer?
Chalk is impure calcium carbonate. the amount of calcium carbonate present can be determined by hydr...
Questions

History, 30.06.2019 00:30

History, 30.06.2019 00:30




English, 30.06.2019 00:30



English, 30.06.2019 00:30

Mathematics, 30.06.2019 00:30



Mathematics, 30.06.2019 00:30

Spanish, 30.06.2019 00:30



Mathematics, 30.06.2019 00:30



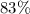 .
.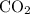 gas are released?
gas are released?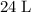 under room temperature and pressure (r.t.p,
under room temperature and pressure (r.t.p, 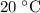 ,
, 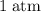 .) That's the same as
.) That's the same as 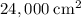 .
.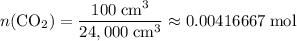 .
.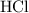 is in excess. How many moles of
is in excess. How many moles of 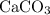 formula units will produce that
formula units will produce that 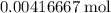 of
of 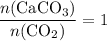 .
.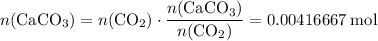 .
.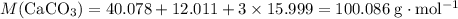 .
.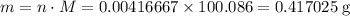 .
.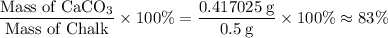 .
.

